Wednesday, June 09, 2004
Programs from the past
 Rooting around in the mustier corners of my harddrive,
I came across this program I had written nearly a decade ago while attending
FAU. At the time, I was a Computer Science major, working in the Math Department for two
Ph. D.s, one out of the Psychology
Department and one from the Center for Complex Systems, both of which
were studying biophysics.
Rooting around in the mustier corners of my harddrive,
I came across this program I had written nearly a decade ago while attending
FAU. At the time, I was a Computer Science major, working in the Math Department for two
Ph. D.s, one out of the Psychology
Department and one from the Center for Complex Systems, both of which
were studying biophysics.
Not that I understood anything of what they actually did; all I did (besides keeping a few computers up and running) was write programs to their specifications.
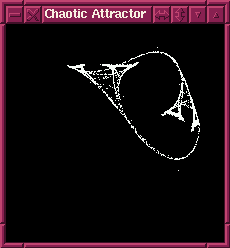
The program I found was one of three I wrote dealing with a pair of equations they were studying:
x1 = ((A × y) + B) × x × (1 - x)
y1 = ((C × x) + D) × y × (1 - y)
From what little I remember, I seem to recall this being a form of simulation of two neurons interacting, but I have no idea how to interpret the results; all I know is that it can produce some rather striking images by ploting the results, then feeding those back into the equation, repeating this several thousand times. By changing the constants A, B, C and D you get wildly different results.
This program was had four slider controls that allowed to you vary the constants and updated the result in real time (and was quite impressive to view on the SGI workstation on my desk at school). The second one (written for a particular video card on a PC) would randomly pick A, B, C and D; you could view the previous 16 images and blow any of them up (or save the parameters to disk for later viewing). You could also have it step sequentially through values. This program was actually the backdrop for a BBC interview of one of the doctors I was working for.
The third program I wrote was a bit more complex. Instead of plotting the results of interation through the equations, it instead kept track of the results, and when it detected a loop, it would then save the number of points generated before a loop was detected (some values of A, B, C and D would vasillate between two or three points, while other values of A, B, C and D would never repeat even after 5,000 interations). And it worked its way systematically, varying A through its range of values and keeping B, C and D constant. It would then bump B up, and then run through all values of A, then bump B up, and so on until B hit its upper limit, then bump C up a bit, and so on. It took the better part of a year to run through all values of A, B and C. Then the data was plotted in three dimentions, using time as one of the dimentions (basically, an animation of a rather odd looking two dimentional image) and stored on video tape (which took me the better part of three days making, having to edit about five minutes of video frame-by- frame).
Again, not that I understood what the results where, just that I did it.
I enjoyed the work, and the office space was incredible; there are days when I wish I was still in that office.
Sigh.
Just for a lark, I decided to Google for the doctors I worked for and came across some of their recent work:
A new proprietary de novo peptide design technique generated ten 15- residue peptides targeting and containing the leading nontransmembrane hydrophobic autocorrelation wavelengths, “modes”, of the human m1 muscarinic cholinergic receptor, m1AChR. These modes were also shared by the m4AChR subtype (but not the m2, m3, or m5 subtypes) and the three- finger snake toxins that pseudoirreversibly bind m1AChR. The linear decomposition of the hydrophobically transformed m1AChR amino acid sequence yielded ordered eigenvectors of orthogonal hydrophobic variational patterns. The weighted sum of two eigenvectors formed the peptide design template. Amino acids were iteratively assigned to template positions randomly, within hydrophobic groups. One peptide demonstrated significant functional indirect agonist activity, and five produced significant positive allosteric modulation of atropine-reversible, direct- agonist-induced cellular activation in stably m1AChR-transfected Chinese hamster ovary cells, reflected in integrated extracellular acidification responses. The peptide positive allosteric ligands produced left-shifts and peptide concentration-response augmentation in integrated extracellular acidification response asymptotic sigmoidal functions and concentration-response behavior in Hill number indices of positive cooperativity. Peptide mode specificity was suggested by negative crossover experiments with human m2ACh and D2 dopamine receptors. Morlet wavelet transformation of the leading eigenvector- derived, m1AChR eigenfunctions locates seven hydrophobic transmembrane segments and suggests possible extracellular loop locations for the peptide-receptor mode-matched, modulatory hydrophobic aggregation sites.
Yea, I don't understand it either.
And that's just the abstract. I can't imagine how impenatrable the actual paper is.

![Glasses. Titanium, not steel. [Self-portrait with my new glasses]](https://www.conman.org/people/spc/about/2025/0925.t.jpg)